

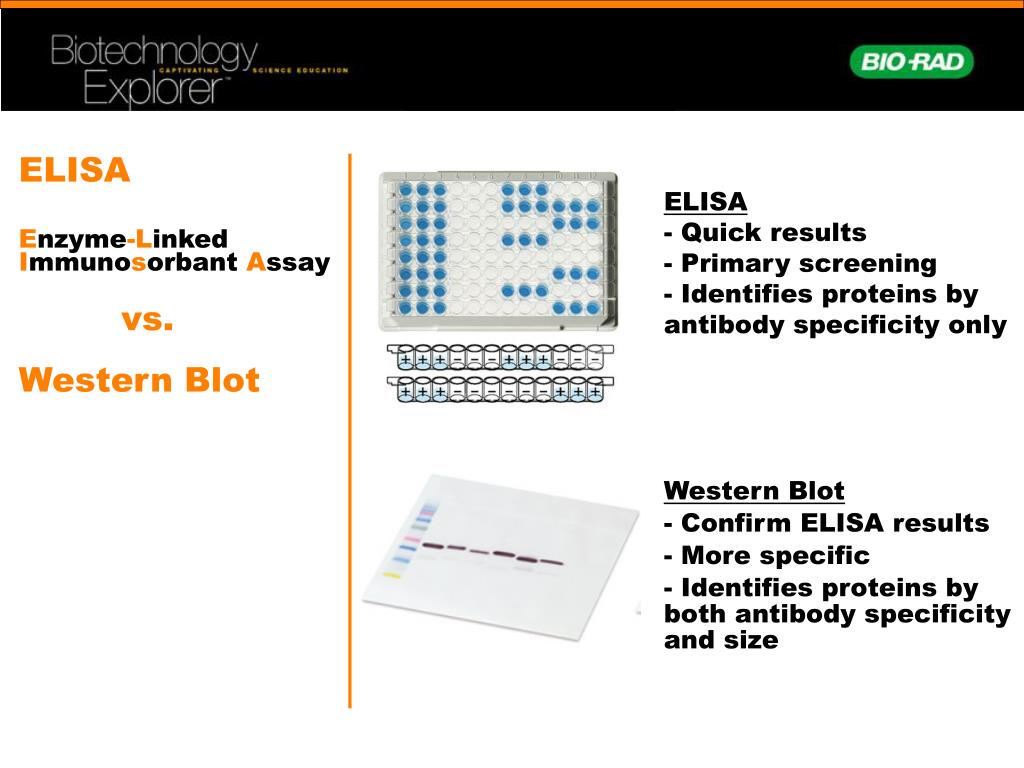
These tests measure antibodies made by the immune system in response to the Lyme infection. The second step uses an immunoblot test, commonly called a “Western blot.” Both tiers–the ELISA and the immunoblot–must be positive for a person to be considered “CDC positive” for Lyme disease.īoth the ELISA and the Western blot are indirect tests - that is, they do not detect the Lyme pathogen itself. The CDC only recommends the second tier if the first step is positive or indeterminate (sometimes called “equivocal.”) If this first step is negative, the CDC says no further testing of the specimen should be performed. The first step uses a procedure called enzyme immunoassay (commonly called the ELISA) or sometimes, indirect fluorescent antibody (IFA). The most common test for Lyme disease is the CDC-recommended two-tier test. While early and accurate diagnosis is the most effective way to avoid more serious illness, the fact is many patients will be improperly diagnosed due to faulty testing. However, studies show that their sensitivity–meaning how well the tests can identify who actually has Lyme disease–can range anywhere from 7.4% to 86.2%. Although the diagnosis of Lyme borreliosis can be based upon clinical symptoms, most doctors will rely on commercially available tests kits.
WESTERN BLOT VS ELISA EQUINE LYME PDF
Users are referred to the electronic PDF version ( )Īnd/or the original MMWR paper copy for printable versions of official text, figures, and tables.Lyme disease is spreading rapidly across the world and is currently the most common tick-borne disease in the United States and Europe.
This conversion might result in character translation or format errors in the HTML version. URL addresses listed in MMWR were current as ofĪll HTML versions of MMWR articles are generated from final proofs through an automated process. Provided as a service to MMWR readers and do not constitute or implyĮndorsement of these organizations or their programs by CDC or the U.S.ĭepartment of Health and Human Services. References to non-CDC sites on the Internet are Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration 2019. FDA clears new indications for existing Lyme disease tests that may help streamline diagnoses. CrossRef external icon PubMed external icon Collection and characterization of samples for establishment of a serum repository for Lyme disease diagnostic test development and evaluation. Notice to readers: recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Washington, DC: Association of State and Territorial Public Health Laboratory Directors 1994. In: proceedings of the Second National Conference on Serologic Diagnosis of Lyme Disease October 27–29, 1994 Dearborn, MI. Association of State and Territorial Public Health Laboratory Directors.Clearance by FDA of the new Lyme disease assays indicates that test performance has been evaluated and is “substantially equivalent to or better than” a legally marketed predicate test. The modified methodology uses a second EIA in place of a western immunoblot assay. On July 29, 2019, FDA cleared several Lyme disease serologic assays with new indications for use based on a modified two-test methodology ( 4). To assist serologic test developers, CDC has made available, with support from NIH, a comprehensive panel of sera from patients with various stages of Lyme disease and other conditions, as well as healthy persons ( 3). Regarding the development of future tests, the report advised that evaluation of new serologic assays include blind testing against a comprehensive challenge panel, and that new assays should only be recommended if their specificity, sensitivity, and precision equaled or surpassed the performance of tests used in the recommended two-test procedure. The conference proceedings recommended a two-test methodology using a sensitive enzyme immunoassay (EIA) or immunofluorescence assay as a first test, followed by a western immunoblot assay for specimens yielding positive or equivocal results ( 1, 2). In 1994, the Association of State and Territorial Public Health Laboratory Directors, CDC, the Food and Drug Administration (FDA), the National Institutes of Health (NIH), the Council of State and Territorial Epidemiologists, and the National Committee for Clinical Laboratory Standards convened the Second National Conference on Serologic Diagnosis of Lyme Disease ( 1). Lyme disease is a tickborne zoonosis for which serologic testing is the principal means of laboratory diagnosis.


 0 kommentar(er)
0 kommentar(er)
